Abstract
The gut microbiota plays an important role in maintaining intestinal homeostasis by regulating the maturation of the mucosal immune system, which constitutes an immune barrier for the integrity of the intestinal tract. In recent years, the role of the human GI microbiota in graft-versus-host disease (GVHD) and other outcomes after allogeneic hematopoietic cell transplantation (HCT) has been increasingly evaluated in observational studies. However, there have been limited interventional trials specifically designed to alter the microbiota of HCT recipients. CBM588 (clostridium butyricum MIYAIRI 588) is a novel Live Biotherapeutic Product (LBP) that produces short chain organic acids, mainly butyric acid, which plays a key role in the maintenance of colonic homeostasis by regulating fluid and electrolyte uptake, epithelial cell growth, and inflammatory responses. In this pilot trial (NCT03922035) we sought to determine the safety, feasibility, biologic activities, and preliminary efficacy of CBM588 in HCT recipients.
Patients age ≥18 years, scheduled to undergo HCT from an 8/8 or 7/8 matched related/unrelated donor with reduced intensity conditioning (RIC) were eligible. Following the patient safety lead-in (SLI; n=6), 30 patients were randomized (1:1 ratio) to receive either standard peri-transplant supportive care alone (control arm) or with CBM588 (treatment arm, open label) at the fixed dose of 160 mg orally (2x/day) from day -8 or hospital admission until day +28 or discharge (figure 1). Patients received prophylactic antibiotics per intuitional SOPs. Study objectives were to evaluate the safety/feasibility of CBM588 (Primary), and to compare the incidence and severity of adverse events (AE), HCT outcomes including GVHD, and gut microbiome diversity between the Treatment and Control arms. Feasibility was defined as the ability to consume CDM588 for 14 days during the SLI phase. For microbiome analysis, we isolated DNA from weekly collected stool samples, and amplified the V4 region of the bacterial 16S rRNA gene from each total DNA sample.
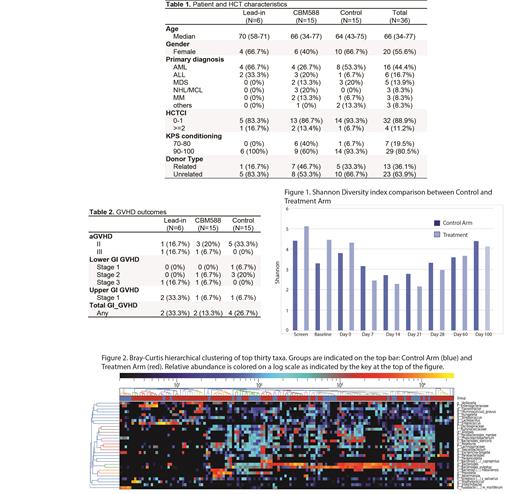
Between April, 2018 and January, 2020, we enrolled 36 patients (20 were female) at the median age of 66 years (range: 34-77). The indication for HCT was Leukemias (n=22), MDS (n=5), lymphoma (n=3), myeloma (n=3), or other (n=3). All but one patient received fludarabine/melphalan-based RIC and tacrolimus/sirolimus-based GVHD prophylaxis. Graft source was peripheral blood stem cell from a matched related (n=13) or unrelated (n=23) donor (Table 1). One patient assigned to the Treatment arm declined to receive CBM588 before the first dose; but remained on the study with clinical data/biospecimen collections and safety/feasibility/biologic endpoints were analyzed as treated for this patient. All the other patients who were assigned to the treatment arm (n=21, including the patients in SLI segment) were able to take the prescribed study drug; with the median 52 doses (range: 0-55), and 19 of 21 subjects (90.5%) consumed at least 14 days of the study drug. There were no serious adverse events (SAE) related to CBM588. The overall AEs and infection- or GI-specific AEs were similar between the Treatment and Control arms. All but one patient (who died of sepsis in the Control arm - on day 8) engrafted with a median of 15 days for neutrophils. The 100-day non-relapse mortality (NRM) was 0% in the Treatment and 6.7% in the Control arm. According to the intent-to-treat principle, acute GVHD (grade 2-4) was observed in 4 of 15 patients in the Treatment arm and 5 of 15 in the Control arm. The lower GI GVHD was seen in 2 patients in the Treatment and 4 in the control arm. As treated analyses showed the overall grade 2-4 GVHD in 3 of 14 (21.4%) with the use of CBM588 and 6 of 16 (37.5%) without CBM588 (one case of lower GI GVHD with CBM, 5 cases without; (Table 2). The Shannon Diversity Index was similar between the two groups at each time point tested. (Figure 1). However, had favorable microbial profile was detected as the pathogens Enterobacteriaceae, Clostridium baratii, and Clostridiodes difficile were reduced in the treatment group. (Figure 2)
In summary, our data demonstrate the feasibility and safety of CBM588 administration during the peri-transplant period, which was associated with an intended biologic impact on the gut microbiome, and an early favorable sign of GI-GVHD incidence and HCT outcomes in this older population who underwent RIC HCT.
Dadwal: Astellas: Speakers Bureau; Aseptiscope: Consultancy; Shire/Takeda: Research Funding; AlloVir: Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Other: Investigator; Karius: Other: Investigator. Pullarkat: AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees; Amgen, Dova, and Novartis: Consultancy, Honoraria. Al Malki: CareDx: Consultancy; Neximmune: Consultancy; Hansa Biopharma: Consultancy; Rigel Pharma: Consultancy; Jazz Pharmaceuticals, Inc.: Consultancy. Ali: Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Speakers Bureau; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees. Artz: Radiology Partners: Other: Spouse has equity interest in Radiology Partners, a private radiology physician practice. Stein: Amgen: Consultancy, Speakers Bureau; Celgene: Speakers Bureau; Stemline: Speakers Bureau. Budde: Roche: Consultancy; BeiGene: Consultancy; IGM Biosciences: Research Funding; Merck, Inc: Research Funding; Gilead: Consultancy; AstraZeneca: Research Funding; Mustang Bio, Inc: Research Funding; Novartis: Consultancy; Amgen: Research Funding. Popplewell: Hoffman La Roche: Other: Food; Pfizer: Other: Travel; Novartis: Other: Travel. Marcucci: Novartis: Other: Speaker and advisory scientific board meetings; Agios: Other: Speaker and advisory scientific board meetings; Abbvie: Other: Speaker and advisory scientific board meetings. Forman: Lixte Biotechnology: Consultancy, Current holder of individual stocks in a privately-held company; Mustang Bio: Consultancy, Current holder of individual stocks in a privately-held company; Allogene: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal